Background:
Heat generation in exothermic Nitration reactions will decompose organic materials with explosive violence! This property is well respected by nitration practitioners and consequently industrial batch reactors are limited to 6000L volumes with long batch cycles.
Reaction:

Experimental:
Side-by-side chemistry was performed comparing a 250ml batch autoclave and a 5ml continuous MicroFlo™. The MicroFlo™ configuration is designed to maximize mixing and heat transfer in a continuous flow enabling exothermic reactions to be run with precise control. High heat transfer rates are obtained using a 3D printed reaction chamber contained within a plate and frame heat exchanger, Figure 1.
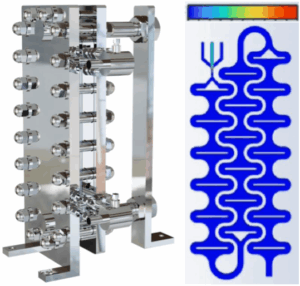
Figure 1: MicroFlo™ reactor assembly (left) and reaction chamber modeled with CFD to show complete mixing of three (3) immiscible fluids (right). Mechanical design limits: 100bar, 350 ⁰C, 100 L/hr.
Results:
Testing indicates the MicroFlo™ can safely perform nitration chemistry at much harsher conditions and consequently, higher production rates and with greater product flexibility. Operating temperatures between 10–20 ⁰C and diluted solutions were required to avoid exothermic runaway in batch however the MicroFlo™ was safely operated at 60-70 ⁰C and with a non-diluted (neat) reaction mixture lowering the reaction time from 3 hours to 5 min, Table 1.
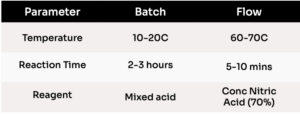
Table 1: Batch and MicroFlo™ results for the nitration of an aromatic substrate. The MicroFlo™ reactor increased production rates by operating with higher temperatures and neat reagents without exothermic runaway.
Conclusions & Commercial Impact:
This study demonstrates the MicroFlo™ significantly improves heat transfer rates when compared to traditional batch reactors enabling highly exothermic nitration chemistry to be performed with high production rates in neat solutions. This results in several process improvements including: higher profitability due to greater throughput, lower OPEX by simplifying downstream separation, lower OPEX by eliminating the solvent feed stream, greater process flexibility since the same equipment can perform more exothermic di and tri nitration reactions, and greater process safety since the process consumes the reactant mixture in real time eliminating large inventories of energetic molecules.
Recent Posts
Manufacturing Compliance: Monitor and Reduce VOC Emissions Effectively
As companies strive to meet environmental standards, VOC (Volatile Organic Compounds) abatement is a critical focus area, particularly for the manufacturing industry. Staying compliant with local and national regulations is…
Read MoreUnderstanding Catalyst Deactivation: How Characterization Can Identify the Root Cause
AbstractCatalyst deactivation is a common challenge in many catalytic processes, but by identifying the root causes, taking appropriate corrective actions, and utilizing advanced characterization techniques, it is possible to maintain…
Read MoreChallenges in Catalyst Regeneration and How to Overcome Them
Learn the techniques for effective catalyst regeneration and how it can optimize your operations. Read now to enhance your industrial processes!
Read More



